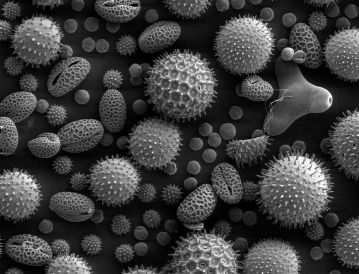
Plants have sex. While flowers, cones, and fruits– basically the vaginas and uteruses of the plant world– feature prominently in human cultures, much of the actual, er, act of plant sex is invisible to us. As northerners dig out from under record-breaking snowfalls and eye the ground for the first crocuses and robins, folks to the south are already beginning to experience a trademark of spring (and the male equivalent in the sex lives of plants): pollen season. The microscopic powder produced by seed-bearing plants are not sperm, per se, but are in fact microgametophytes, or sperm-producing cells. The word “microgametophyte” packs a handy definition for those up on their ancient Greek: “micro” means tiny, “gamete” is a combination of “husband” and “wife,” and “-phyte” refers to anything plant-related. Basically, pollen is a sperm delivery system. If a pollen grain “gets lucky,” that is, it meets the flowers or cones of its species, it germinates, creating little tubes to deliver the sperm to the ovule, which is the female gametophyte. That’s plant sex, in a nutshell.
But what about the pollen that doesn’t make it to ovules, the losers in the world of plant procreation? What happens to all the unsuccessful pollen?
To answer that, you have to start with a different question: why do plants make so much pollen in the first place? As anyone with seasonal allergies knows, pollen gets everywhere, forming a fine yellow powder that coats cars and windows, and is even visible on the surface of lakes in regions with lots of conifers (the champions of pollen productivity). You may be tempted to think that all that pollen is a big waste, but it’s actually part of a very successful evolutionary strategy. Genetic variation is a good thing (cue the theme song for Deliverance), and so plants have evolved strategies to share their genetic material with as wide a range of potential mates as possible. Tobler’s First Law of Geography states that things that are closer to you tend to be more related, and so it follows that getting your pollen to the flowers and cones of other, faraway plants is a smart strategy if genetic diversity is the goal. Plants disperse their pollen in one of three ways: wind, animals, or (for a few aquatic plants) water. Of these, wind is the best method for getting your pollen to faraway plants, as long as there are enough potential individuals to reach. This strategy is especially common for weedy species (like ragweed) and for trees in the boreal and temperate zones (the tropics tend to have many more specialized animal pollen dispersers). A large reason for this is the fact that the trees in the temperate regions of the northern hemisphere are windswept in spring, and there are usually only a few, common tree species present (so an inanimate transport method is especially effective). Pollen can be transported quite long distances– in fact, Ephedra pollen from the American southwest has been found in sediment cores as far away as Ireland! Because this is a post about failed plant sex, we’re going to stick with the wind-pollinated (“anemophilous,” or wind-loving) plants, which produce those copious amounts of pollen that are so ubiquitous–and irritating for those with allergies– in the flowering season.

Pollen floating on a lake, where wind and currents have pushed it close to shore. This pollen has failed at sex, but wins at science! Image from Wikimedia Commons.
To get back to the original question, much of the pollen that doesn’t make it to other plants ends up in lakes and bogs. While people have been admiring pollen grains under a microscope since the mid-17th century, the idea that all that excess pollen might be ecologically useful didn’t take hold until a 1916 talk by Lennart von Post. von Post was a peat scientist working for the Swedish Geological Survey, and is known as the Father of Palynology (the study of pollen, from the Greek for “the study of things sprinkled around”). He was the first person to appreciate the fact that the changing amounts of different kinds of pollen preserved in the peats could essentially be read as an archive, telling the story of vegetation around the bog. He realized that fluctuations in the pollen of various plants could be interpreted as signals that a new species had migrated into the region, flourished when its climate was optimal, or died when it was not, to be replaced by other plants more suited to the local conditions at the time (you can see the first-ever published pollen diagram here).
How does this work, exactly? For starters, lakes and bogs act as giant collectors, accumulating debris from the organisms in and around the surrounding watershed. This material (pollen, plant parts, charcoal, insect bits, and other debris) settles to the mud at the bottom of the lake or the top of the bog, where the cold, oxygen-depleted water of the former or the acidic conditions of the latter preserve the material through time. As this accumulates, it forms layers in the mud, with the deepest being the oldest. In other words, every lake and bog you see on the landcsape is an archive of environmental change through time. The bigger the lake or bog, the broader the “source area” of the pollen, and the greater the scale of the landscape history you’re able to reconstruct with pollen. In areas where the ice sheets reached their maximum extent, those archives may go back more than twenty thousand years; in the rift lakes of Africa, the records can span hundreds of thousands of years of earth’s history (though these are considerably harder to acquire).
Fortunately, pollen is incredibly resilient stuff, protected by a coating of one of the most resistent biopolymers known in nature, sporopollenin. In fact, sporopollenin is so resilient that its chemical makeup is not entirely known; it’s so effective at resisting breakdown by other chemicals that the usual techniques fail to determine its composition. Corrosive acids and bases have little effect on sporopollenin, though strong oxidizers like bleach will destroy pollen in minutes. The sporopollenin coating is handy when it comes to protecting the pollen on its long-distance journey to other plants, but it also helps preserve the pollen in the sedimentary archive. Thousands, or even millions of years after its parent plant has died, pollen-rich cores of mud and peat– like an ice core– can be extracted by paleoecologists like me. You read that right– pollen is even being extracted from shales–hard rock– from the Miocene (23 million to 5.3 million years ago), and scientists are testing its isotopic make-up to understand the evolution of grasslands and different photosynthesis strategies in grasses.

A core segment from Silver Lake, Ohio; older, silty clay associated with cooler and drier climates on the left transitions to organic-rich mud, right. This mud will have to be dissolved with a lengthy series of chemical digestions to extract the pollen contained within. Photo by Jacquelyn Gill.
Once the mud is taken back to the lab, sporopollenin’s resiliency shines. The trick to isolating pollen grains from everything else in your mud core– smooshy bits of leaves and other organic parts, sand, wind-blown dust, clay, etc.– is a series of chemical “digestions” designed to dissolve everything except those little microgametophytes of interest. Pollen analysts subject the samples to a careful series of treatments, designed not only to break down the unwanted material, but also to remove organic acids and other components of the mud in order to prepare it for further treatments down the chain. Strong acids and bases are added in succession, dissolving organic matter, carbonates, and sands (!), leaving a small fraction of the original cube of mud each sample starts out as. If you’ve done everything right, you’ve got mostly pollen at the end. Dab a little of it on a microscope slide, magnify it to four hundred times its actual size, and you become a paleo-detective, reconstructing the landscape scene from the pollen clues left behind by long-dead plants. In fact, palynology has become a useful tool in forensics, since it can provide valuable information about where bodies may have been at particular times (including Ötzi the Ice Man).
Pollen grains are quite distinctive under a microscope, which is a big reason for their usefulness. Not all pollen can be identified to the species level, though. For some trees, like oaks, the morphology is too variable within species, too similar to other species, or both. Under a microscope, oak pollen has the gestsalt of “oak” but an analyst can’t tell the difference between, say, a red oak and a white oak. Still, the amount of information recorded in the pollen record is remarkable, particularly when it comes to global change research. Pollen analysis has been used to reveal how plants responded to cycles of severe drought in the Great Plains, has captured the widespread deforestation immediately following the KT impact event that killed the dinosaurs, shown the persistence of the Amazon rainforest during the colder, drier climates of the last ice age, elucidated the pre-settlement vegetation on Easter Island, linked the end of the Age of Pyramids in ancient Egypt to widespread drought, and shown how climate change and the extinction of the ice age herbivores caused the formation of completely novel communities of plants (my research).
When you put many of these “fossil” pollen records together, you can map out how trees have shifted their ranges and abundances in response to past climate change, which is one of the primary clues about how well species may be able to keep pace with climate change in the future. Check out these cool animations of mapped tree distributions over the last 21,000 years to get the idea. According to the rates observed in the North American pollen record, tree populations were able to spread from 100 to 1000 meters a year, which is an order of magnitude faster than predicted based on observed seed dispersal distances today. This disagreement is known as “Reid’s paradox,” and is an active area of research. The discrepancies between modern observations and the pollen record may be due to the fact that it’s difficult to observe long-distance seed dispersal in the field, and so it may be more common than we think. Or, there may have been “cryptic” populations, or “refugia,” hanging out in suitable microhabitats close to the ice sheets, acting as a sort of advance scout force ahead of the migratory front. These populations may have been so small as to essentially be invisible in pollen records at larger scales, which is why they’ve been unappreciated in the past. Another hypothesis is that paleo-seeds may have had help from birds or extinct megaherbivores, which is bad news for plants looking to hitch a ride in today’s fragmented landscape. All of these hypotheses can be tested with a combination of pollen analysis and other tools, like phylogeography (the geography of genetics) and computer modeling.
Ecologists and conservationists are increasingly turning to the lessons from pollen –from failed plant sex– for a long-term perspective on issues like conservation baselines, ecosystem resiliency, disturbance, and climate change. With apologies to those who suffer from seasonal allergies, we have a lot to thank the plants of the past for. Without a reproductive strategy that essentially relies on prodigious ejaculation, we’d know a lot less about what North American forests looked like before Europeans cut them down, or what extinct moas ate. Not bad for for a bunch of failures.
Edited to clarify some details on the evolutionary advantages of wind pollination in temperate and boreal trees, which are still somewhat oversimplified in this post. Thanks to evolutionary ecologist Tom Givnish, who taught me Advanced Plant Community Ecology back at UW Madison, for feedback!
Categories: Explainers Research

Hi, Jacquelyn! I stumbled across your blog a while back and enjoyed the scientific detail you write with. 😀 So much so that I thought I’d pass on an award which had been given to me: The Liebster Award. Take a moment, if you can find the time, to accept this nomination and pass it on to others you feel deserving of the same recognition. ~DM Peachey from apeachintime~
LikeLike
Very Good Article!Pollen to honey, bless the bees.
LikeLike
Ah, those long hours of poring over “palynographs” come to memory…
LikeLike
Reblogged this on Oyia Brown.
LikeLike
Wow, this was very informative! I have learned a lot from it.
LikeLike
My friends and I recently made a short film on global climate change, i think you’d like it: http://youtu.be/6ZihKHKY0N8
LikeLike
This sort of thing is really useful to archaeology, too – you can work out a lot about the surrounding environment of past societies at different times, and potentially answer questions like how fertile the land was, what crops were produced, how the environment changed after people settled in an area, etc.
LikeLike
Interesting, Don’t think I will be able to look at plants and nature the same again this summer lol 😀
LikeLike
Thanks! Then my work here is done. 🙂
LikeLike
Oh it Is! I assure you 😀
LikeLike
“what they said” (everyone so far). i ALWAYS like it when i read and learn something in a manner which makes it not only digestible, but interesting.
LikeLike
Very well done! You totally mastered sharing/teaching a great deal of information; with a brilliant mixture of technical and laymans’ terms. And this will suffice for my “learn something new everyday” item. 2 thumbs UP
LikeLike
Thank you! That was my goal, and your feedback is really quite helpful! I’m glad you enjoyed it!
LikeLike
Reblogged this on .
LikeLike
Reblogged this on Curated Larning and commented:
Live and learn, Bob. Live and learn.
LikeLike
Excellent article but brings up bad memories cause I’m allergic :p
LikeLike
Thanks, and sorry about that! At least now you can know that your suffering is for a good cause, right? 🙂
LikeLike
Nice article. Tough to throw in so many technical terms and still make it readable but you managed to do it. You also make a lot of factual information reachable. Thanks for the post.
LikeLike
Thank you! I was very deliberately trying to introduce some scientific terms but still make it accessible, and so I’m very glad that it worked. Your feedback is really valuable to me as a scientist who cares about communication!
LikeLike
Who would have thought that plant sex was so exciting! Can extinct plants be brought back from these sperm laying around for thousands of years waiting to spread their seed?
LikeLike
Good question! Sadly, while the shell of pollen (made from Sporormiella) is very resilient, the reproductive part inside is not as hardy. That has mostly decayed by the time I get to it. However, I do have colleagues looking at the DNA in ancient pollen grains to study the populations of trees in the past, which helps get at that migration question I mentioned. They’ve found that the older you go, the rarer DNA is in pollen, because of the preservation problem. In some cases, less than one out of a hundred pollen grains have any measurable DNA at all! So, that’s not a likely solution. You might be interested in a post I wrote about some seeds from an ice age squirrel’s snack that were germinated, though!
LikeLike
Nice post!
LikeLike
Thanks, John!
LikeLike
Really nice post. My only quip is that maybe you should have written “animals” instead of “insects.” What about the birds (e.g. hummingbirds), bats (especially important in the tropics), and occasional terrestrial mammals (e.g. lemurs) and lizards?
LikeLike
Oh, good call! I totally knew that, too! I was showing my North American pollen bias. Fixing now.
LikeLike
This is great! I was just wondering yesterday how exactly pollen is isolated and counted for such data. Thanks for writing this.
LikeLike
Happy to oblige! I’m glad you enjoyed it.
LikeLike